[Startup Spotlight]: Pioneering Next-Gen Intracellular Therapeutics with CyGenica
If you are someone who is interested/building/an expert in the Life Science startup ecosystem in India and/or the US, I would love to connect on LinkedIn. Every couple of weeks, I highlight founder stories or talk about the infrastructure for life science in India with comparisons drawn to the US.
CyGenica, an innovative biotech startup, was founded by Dr. Nusrat Sanghamitra, an Indian scientist, entrepreneur, and motivational leader who serves as its CEO and CSO, and Sk Fazlul Haque Krishnan, the Director of Operations responsible for executing key policies, strategies, and milestones. The company is revolutionizing the field of intracellular therapeutics with its groundbreaking protein-based drug delivery platform, GEENIE. This versatile and scalable platform simplifies drug delivery into cells, enhancing precision and efficiency while paving the way for a new era in pharmaceutical treatments. Leveraging unique technical capabilities and strategic partnerships with global biopharmaceutical companies, CyGenica has positioned itself as a key player in the life sciences sector, attracting significant interest from US-based investors and partners. The company remains committed to maintaining global standards of credibility and quality in its research and development efforts.
CyGenica’s Platform: Redefining Drug Delivery with Key Differentiators
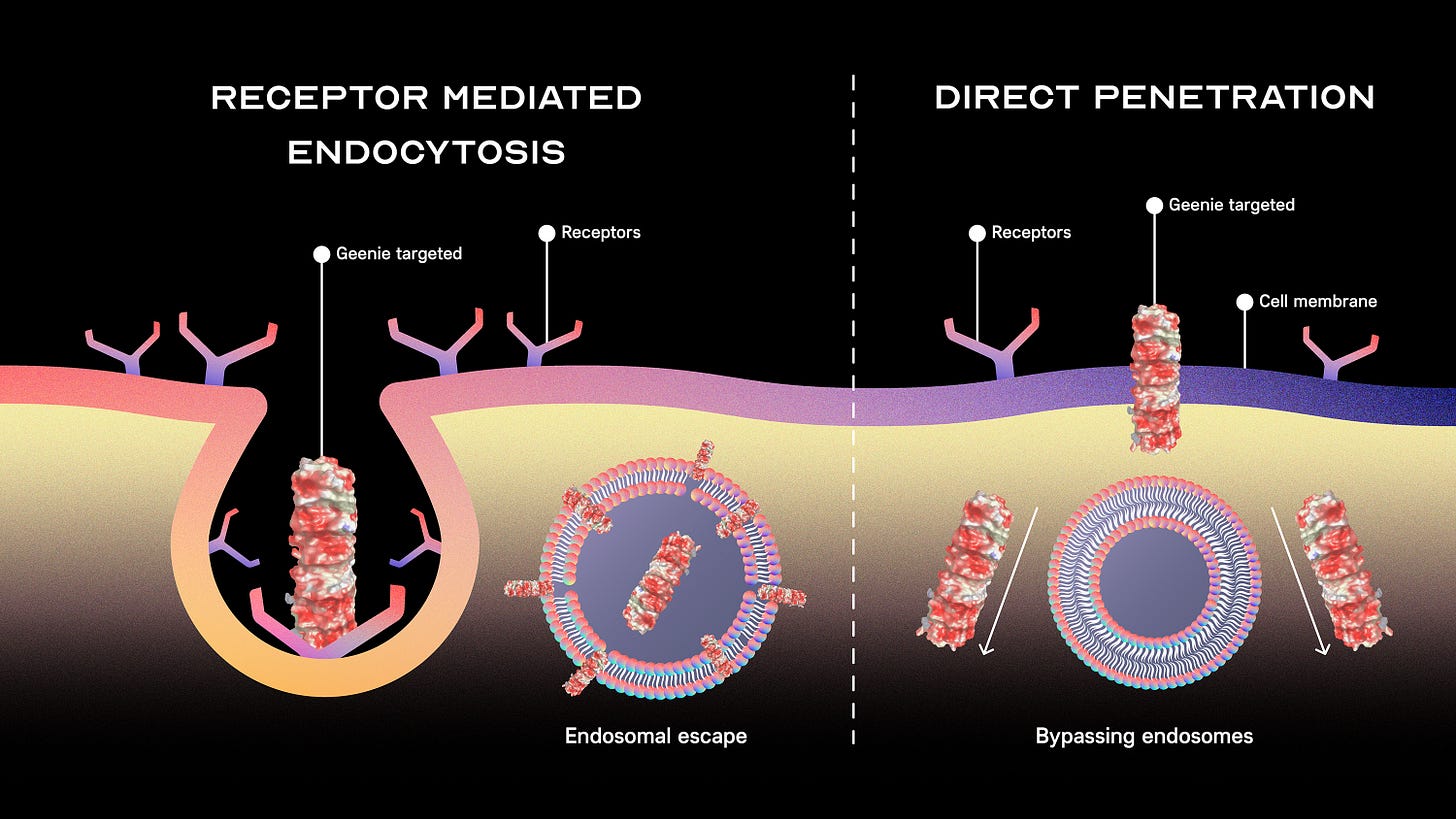
CyGenica's platform, GEENIE, centers on a novel protein nano needle that directly penetrates the cell membrane, bypassing the endosomal pathway—a complex process that other delivery systems often struggle to overcome . Traditional delivery systems, often face limitations with endosomal escape, achieving only about 50% efficacy. In contrast, CyGenica’s platform ensures more effective therapeutic delivery directly into the cell’s interior, an advantage that has drawn attention from pharmaceutical companies and investors alike, especially since publications on endosomal escape has doubled in the past ~ 7 years.
Other standout qualities of CyGenica’s platform include its simplicity, robustness, and recombinant protein base, each of which adds to its appeal as a scalable and cost-effective solution. The platform’s high stability—across varying melting temperatures and pH levels—enhances its robustness and ease of manufacture. This simplicity is essential not only for laboratory-scale production but also for larger commercial manufacturing. By using recombinant proteins, CyGenica’s platform achieves a high level of reproducibility and cost efficiency, making it an attractive long-term investment for both its partners and investors.
Moreover, the platform’s versatility in delivering a diverse range of therapeutic cargos—small molecules, RNA-based drugs, CRISPR-Cas systems, and proteins—broadens its applicability across various treatment areas. The ability to deliver treatments directly across the blood-brain barrier has been particularly appealing to US-based companies interested in central nervous system (CNS) therapeutics, which represents a high-demand and high-impact area in modern healthcare.
Additionally, they have achieved a major milestone with the U.S. Food and Drug Administration (USFDA) granting Orphan Drug Designation for its novel drug conjugate designed to treat Glioblastoma Multiforme (GBM), an aggressive and challenging form of brain cancer. This landmark approval not only highlights the promise of CyGenica’s groundbreaking intracellular delivery platform, GEENIE, but also paves the way for transformative advancements in cancer and rare disease therapies, offering renewed hope to patients in dire need.
Strategic Biopharmaceutical Partnerships: Building on Global Credibility
CyGenica has strategically chosen to work with pharma/biotech companies with a strong global presence and reputation which have European or US origins. This choice addresses the perception bias sometimes associated with Indian-only companies, allowing CyGenica to build on the credibility and quality standards established by globally recognized organizations. Despite conducting much of its experimental work within India, the company leverages the international reputation of its biopharma partners to meet the expectations of US and European clients.
While these partnerships come with increased costs compared to working solely with local Indian biopharma companies, CyGenica believes the higher expense is justified by the enhanced credibility and quality of outcomes. This global partnership strategy has enabled the company to achieve consistency in standards, further enhancing its appeal to US-based investors who prioritize quality, reliability, and regulatory alignment.
By leveraging the resources, knowledge, and infrastructure of global biopharma companies, CyGenica is well-positioned to address the scalability demands that come with growth, without compromising on quality. This careful balance of cost, scalability, and quality has made CyGenica a favored choice among investors looking for reliable, scalable solutions in the drug delivery domain.
Leading the Next Generation of Drug Delivery Innovation
With a delivery platform that redefines industry standards and a strategic focus on global credibility, CyGenica is paving the way for future breakthroughs in precision therapeutics. By partnering with global biopharmaceutical companies known for their international quality and reputation, CyGenica not only ensures top-tier standards in its processes but also showcases a model that other Indian startups can follow. This approach—combining innovative technology with trusted global partnerships—not only enhances scalability but also builds credibility on a global stage, setting a powerful example for emerging Indian companies aiming to expand internationally. As CyGenica continues to grow, it exemplifies how thoughtful strategy, and technological advancement can drive lasting impact in the biopharmaceutical sector.
Reference:
PR Newswire. CyGenica Limited secures USFDA approval for orphan drug designation for novel drug conjugate in Glioblastoma Multiforme treatment. PR Newswire. https://www.prnewswire.com/news-releases/cygenica-limited-secures-usfda-approval-for-orphan-drug-designation-for-novel-drug-conjugate-in-glioblastoma-multiforme-treatment-301923349.html